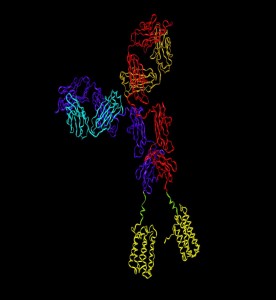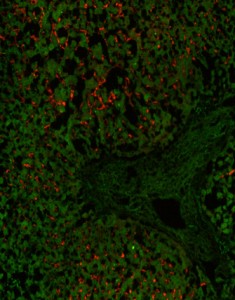
Staining with s-TCR-L on HLA-*02 (þ) liver biopsy with a larger field of view to include liver architecture (green). Envelope antigen expressing hepatocytes were detected by sTCR-L (red). sTCR-L bound exclusively to infected hepatocytes and not to endothelial or stromal cells of the hepatic triad, containing the hepatic artery, vein, and bile ducts (white arrow). Microscopy pictures have been normalized to the green channel.
Research Fellow
Research Overview
Vertical (mother-to-child) transmission is the major cause of hepatitis B virus (HBV) infection in children in Asia. HBV vertical transmission has been hypothesized to cause a state of immune tolerance to HBV. However, the extent to which the fetal immune system is exposed to HBV antigens and whether this can cause a global alteration of adaptive and innate immunity have never been characterized.
This study aims to gain an understanding of the key components of the immune response to HBV present in cord blood of HBV-infected mothers. We aim to characterize the HBV-specific and global innate and adaptive immune profile using molecular and cellular methods. The characterization of the HBV immune response in utero will provide information about the cause of HBV chronicity in Asian patients and in the management of baby born to HBsAg+ mothers.
Senior Research Fellow
My research interests are in understanding the role of the immune microenvironment in disease settings. I have previously studied the role of the immune microenvironment in tumour progression and in solid organ transplantation, primarily using rodent models. In collaboration with the Humanized Mouse Unit at the Institute of Molecular and Cell Biology, I am currently characterizing a humanized mouse model of hepatitis B virus (HBV) infection. The aim is to better understand the phenotypic and functional changes in the immune system during chronic HBV infection in order to develop a model system for testing potential therapies.
Alfonso Tan Garcia
Research Overview
Specific projects:
- Investigation of the viral and immunological events that lead to T cell exhaustion in chronic HBV patients (Nina Le Bert, Laura Rivino).
- Characterization of the immune correlates of liver damage in chronic HBV patients following withdrawal from anti-viral therapy (Maggie Van den Berg, Nina Le Bert, Laura Rivino)
- Characterization of HBV-specific T cell responses in acute-resolved HBV patients and in healthy individuals following HBV vaccination (Kamini Kunasegaran, Christine Tham, Laura Rivino)
- Characterization of the hierarchy of the CD8+T cell response in acute versus chronically infected hepatocytes (Zack Ho, Laura Rivino)
Nina Le Bert
Damien Tan
Nasirah Banu
Kamini Kunasegaran
Christine Tham
Research Fellow
Research Overview
The TCR Therapy Group is particularly interested to develop immune based therapies to reconstitute HBV-specific immunity in patients who are chronically infected or to target tumors expressing HBV antigens in patients with HBV-related hepatocellular carcinoma (HCC) since in these patients, HBV-specific T cells are functionally exhausted or deleted. Our approach is to genetically modify T cells by T cell receptor (TCR) gene transfer to generate new T cell responses that may supplement the deleted or functionally deficient HBV-specific T cells.
HBV-specific TCRs are cloned from T cells obtained from individuals with acute resolved HBV infection (that is likely to be associated with protection) and TCRs of known specificities are transferred to T cells from patients using retroviral transduction or messenger RNA (mRNA) electroporation. The functionality of TCR gene modified T cells to target HCC has been validated in vitro and in vivo in a HCC xenograft mouse model.
We are now working to translate the use of TCR gene modified T cells to the clinic for treatment of HBV-related HCC patients. In addition, we are actively working on engineering T cells that are able to inhibit HBV replication without causing hepatotoxicity, with the goal of using T cells with non-cytopathic antiviral activity for adoptive cell therapy in chronic HBV patients.
Christine Tham
Nasirah Banu
Wu Juan (HCC clinical trial – Research Center for Biological Therapy, Beijing 302 Hospital)
Research Fellow
Research Overview
Chronic hepatitis B virus (HBV) infection affecting more than 350 million worldwide can lead to sever liver diseases including cirrhosis and hepatocellular carcinoma (HCC). In our research projects we are employing different techniques to answer critical questions on virology aspect of HBV infection. One interesting question to answer is how virus production, replication and viral mRNA kinetics might be affected or may affect the immune response to HBV infection using our infection system.
On another aspect, we aim to answer one controversial question on the differences between tumor and Adjacent non-tumor regions in HBV-HCC patients in terms of presence or absence of virus replication or viral antigen presentation levels.
Yang Ning Han
Zack Ho
Erica Ceccarello
Research Fellow
Research Overview
The importance of T cells in the control of HBV infection has been demonstrated in experimental infections of animal models, and also supported by many clinical studies. Following this concept, the most recent therapeutic approaches focuses on adoptively transferring engineered HBV-specific T cells into patients in an attempt to combat infection, in the case of chronic HBV infection, or eradicate HBV-related liver tumours respectively. Understanding the liver microenvironment and how it subsequently affects the behavior of the engineered T cells becomes crucial in order for this therapeutic approach to be successful. My interest hence lies in analyzing how immune effector cells in the liver differ from their peripheral blood counterparts and how HBV infection and progression to hepatocellular carcinoma alters both the liver microenvironment and the resident immune cells. At the same time, in collaboration with Biosym (SMART-MIT), we have developed an imaging-based 3-dimensional microfluidic assay for the investigation of cellular cytotoxicity and dynamics as a pre-clinical test bench to understand the functionality of effector cells in environments similar to that encountered during actual adoptive therapy.
Adeline Chia